Balancing Net Ionic Redox Equations
They gain one or more than one electron and do not lose any protons. These electrolytes are split up and written as individual ions.
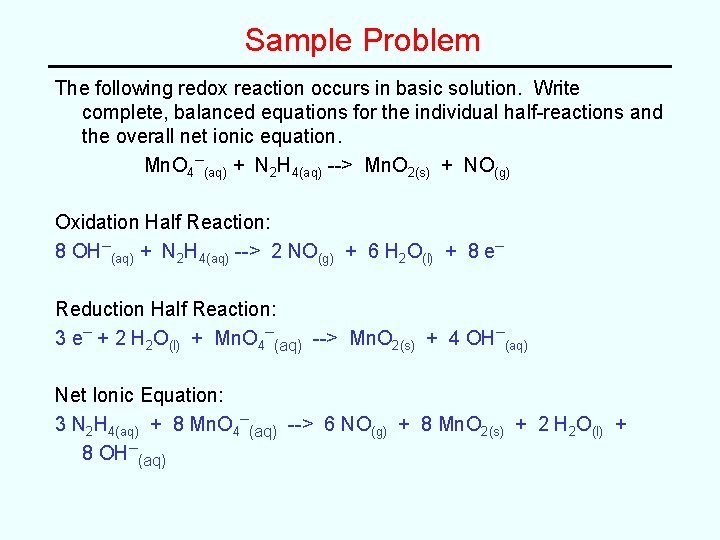
Oxidation Reduction Reactions Redox Terminologyreview Chapter 4 Redox
Heres an example of how to balance the reaction between potassium permanganate and iodide ion in aqueous sulfuric acid to form potassium iodide and manganeseII sulfate.
. The mole 22 26 Concentration and activity 22 27 Organic molecules structure and chemistry 23. Ionic chemicals involve electrolytes which are substances that dissociate into ions when dissolved in polar solvents. Molecular complete ionic and net ionic equations.
Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. While fully ionic bonds are not found in nature many bonds exhibit strong. You did so well on this quiz you could tutor others on how to balance equations.
The same goes for example balancing redox equations in. Redox Oxidation - Reduction. Modification of work by the Italian voiceFlickr.
Some examples of cations are Calcium Ca 2 Potassium K hydrogen H. Predicting Precipitates and Net Ionic Equations Video Take Quiz. Predicting Precipitates and Net Ionic Equations Precipitation Reactions.
Predict the product of the following reaction with a chemical equation calculator. In chemistry the oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero. Constitutes the Net Ionic Equation.
The method is as follows. 648 The potassium problem. Redox reactions Opens a modal.
Anions are negatively charged ions. This is the currently selected item. This isnt always zero.
232 Ionic bonding ions and ionic solids 20. This isnt always zero. Equation balancer is an online tool for balancing chemical equations.
Ionic chemical equations are slightly different from that of a classic case of chemical equations. Balancing chemical equations 1 Get 3 of 4 questions to level up. Lesson 9 - Precipitation Reactions.
This method is mainly being used for balancing redox reactions in acid on the basis of oxidation numbers. Modification of work by vxlaFlickr. 24 Using chemical equations 21 25 Describing amounts of substances.
Balancing for charge means you have the same net charge on both the reactant and product side of the equation. They are formed when non-metal gains the electrons. JEE Mains Chapter wise Practice Questions Last 30 Years - examsnet.
Vedantus online quiz is curated by the best subject matter experts. Topics include properties of solutions acids and bases kinetics equilibrium thermodynamics oxidation reduction ionic and redox equations and electrochemistry. There are Two Methods for Balancing a Redox Reaction Namely.
Formatting them differently bold would help student identify during the learning phase. Net ionic equations for ALL reactions of strong acids with strong soluble bases that form soluble salts and water is. Caracterdesign Getty Images Great job.
The concept of complete and net ionic equations should have been integrated within the precipitation and acidbase reactions instead of standing alone in the balancing equations section. Predicting Precipitates and Net Ionic Equations Video Take Quiz. Modification of work.
This course continues the examination of principles and applications of chemistry that was begun in CHM150. How To Write Net Ionic Equations Step by Step. Therefore they possess a net negative charge.
Take these free online quizzes on maths physics chemistry and enhance your competitive problem-solving skills. Lesson 9 - Precipitation Reactions. Redox flow batteries are a critical technology for large-scale energy storage offering the promising characteristics of high scalability design flexibility and.
Therefore they possess a net positive charge. H OH- à H2O. If youre feeling a bit shaky on all the steps and details you can review the simple method of balancing equationsOtherwise you may wish to review how to balance oxidation-reduction or redox reactions or move on to understanding mole relations.
The most complicated molecule here is C 2 H 5 OH so balancing begins by placing the coefficient 2 before the CO 2 to balance the carbon. Predicting Precipitates and Net Ionic Equations Precipitation Reactions. Balancing the seawater major ion budget 214 65 Minor chemical components in seawater 216.
Complete ionic and net ionic equations Opens a modal 2015 AP Chemistry free response 3a Opens a modal Up next for you.

9 1 Writing Net Ionic Equations Sl Youtube

Balance Redox Reaction Ionic Half Equation Method Youtube
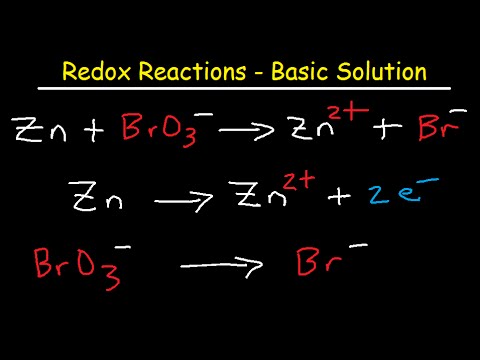
How To Balance Redox Equations In Basic Solution Youtube

Redox Reactions Part 4 Writing Net Ionic Equations Youtube
Oxidation Reduction Reactions Redox
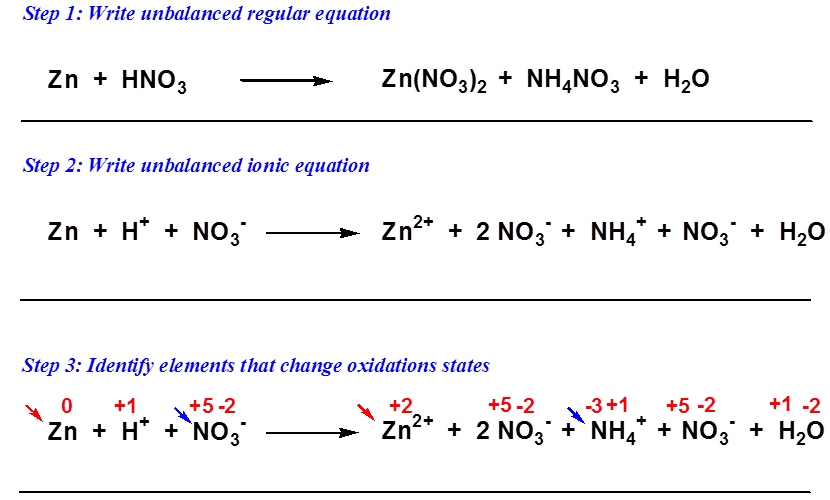
0 Response to "Balancing Net Ionic Redox Equations"
Post a Comment